
PhD student Andrew Rodda and colleagues in biomaterials research at Monash University have been studying a plant-based compound derived from the seeds of the tamarind tree, known as xyloglucan. It can be injected into an injury site as a liquid and gels upon reaching body temperature.
Andrew was able to show in rats that the gel can cause nerve regrowth within an injured brain. His work is being presented for the first time in public through Fresh Science, a communication boot camp for early career scientists held at the Melbourne Museum. Andrew was one of 16 winners from across Australia.
All damage to the nerve cells of the central nervous system—the brain and spinal cord—is considered unrepairable. This leaves sufferers of many diseases and injuries with permanent disabilities—a major economic and social problem worldwide.
The lack of regrowth is due mainly to the toxic environment left behind after nerve death, Andrew says. “Nerve cells are sensitive, and will only grow in the most supportive of environments.”
“After injury, new cells cannot normally penetrate into the empty space left after mass cell death. Cells clump at the edges, forming an impenetrable barrier. This leaves the centre of the wound as a lesion, which contains chemicals that kill growing nerves.”
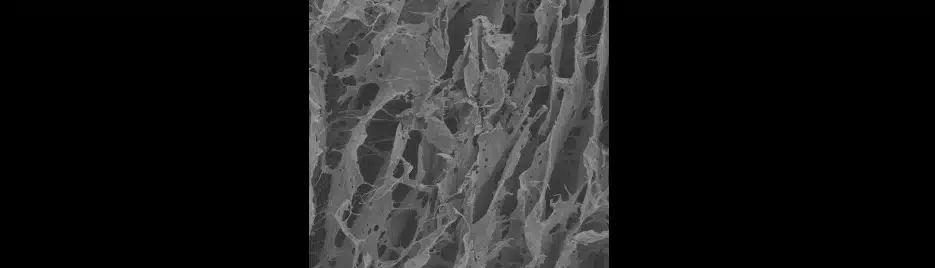
Andrew and his collaborators, however, found that the gel acts as a support structure through which cells can migrate and potentially reattach themselves to the nervous system. “The material provides a temporary scaffold on which new cells can grow and penetrate the lesion.”
In Andrew’s studies the gel was chemically modified to support cell growth and then implanted into a rat brain. Not only did it encourage regrowth in the injured brain, but it also suppressed the post-injury inflammation around the edge of the wound, that goes on killing nerves long after the original damage has been done. Nerves and other cell types then entered and repopulated the empty space filled by the gel.
Significantly, it was the helper-cells known as astrocytes that were the first to move into the implanted gel. These cells secrete beneficial chemicals, which may have helped create an environment in which the delicate nerve cells can survive.
Andrew’s study is part of a worldwide effort to encourage nerve regeneration in the brain and spinal cord. It builds on previous work at Monash to understand and control nerve growth using biomaterials.
Andrew Rodda is one of 16 early-career scientists unveiling their research to the public for the first time thanks to Fresh Science, a national program sponsored by the Australian Government.
Over the course of Fresh Science he is presenting his work:
- in verse at Tech on Tap, part of AMP’s Amplify Festival in Sydney on Monday evening
- over dinner with Australia’s Chief Scientist in Melbourne
- to school students in Melbourne and Bendigo.
For interviews, contact Andrew Rodda on Andrew.Rodda@Monash.edu
For Fresh Science, contact Sarah Brooker on 0413 332 489 or Niall Byrne on 0417 131 977 or niall@scienceinpublic.com.au
For Monash contact Craig Scutt, 03 9903 4844, Craig.Scutt@Monash.edu








 Fresh Science is on hold for 2022. We will be back in 2023.
Fresh Science is on hold for 2022. We will be back in 2023.