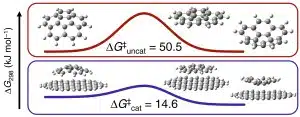
Tiny flakes of graphene can speed up chemical reactions, increasing the efficiency of our smart devices and reducing costs across industries such as mining and pharmaceutics according to Perth researchers.
“Shaped like honeycomb, graphene is 200 times stronger than steel, as transparent as glass and can conduct electricity better than copper,” explains Dr Amir Karton, an ARC Discovery Early Career Researcher Award (DECRA) Fellow at the University of Western Australia.
“Using graphene to more efficiently convert resources that we take from the world around us means less processing, less waste, and will ultimately make end products cheaper for businesses and consumers.”
Made out of a 2D sheet of carbon atoms, graphene won its discoverers a Nobel-prize in 2010.
“Graphene is an exciting material to work with in nanotechnology because its 2D structure and unique chemical properties make it a promising candidate for new chemical applications,” says Amir.
The first two-dimensional structure of carbon ever discovered, graphene’s unique chemical properties make it a promising candidate for applications from flexible, transparent touchscreens for smart devices to renewable energy.
Amir presented his research at Fresh Science WA 2015. Fresh Science is a national program that helps early-career researchers find and share their stories of discovery. Over 20 early-career researchers nominated for Fresh Science WA, which was held at the Western Australian Museum (training) and the Brisbane Hotel (public challenge event) and was supported by the Western Australian Museum, Curtin University, Edith Cowan University, Murdoch University, the University of Western Australia and the University of Notre Dame, Australia.
Contact: Amir Karton, The University of Western Australia, amir.karton@uwa.edu.au
http://www.chemtheorist.com





 Fresh Science is on hold for 2022. We will be back in 2023.
Fresh Science is on hold for 2022. We will be back in 2023.